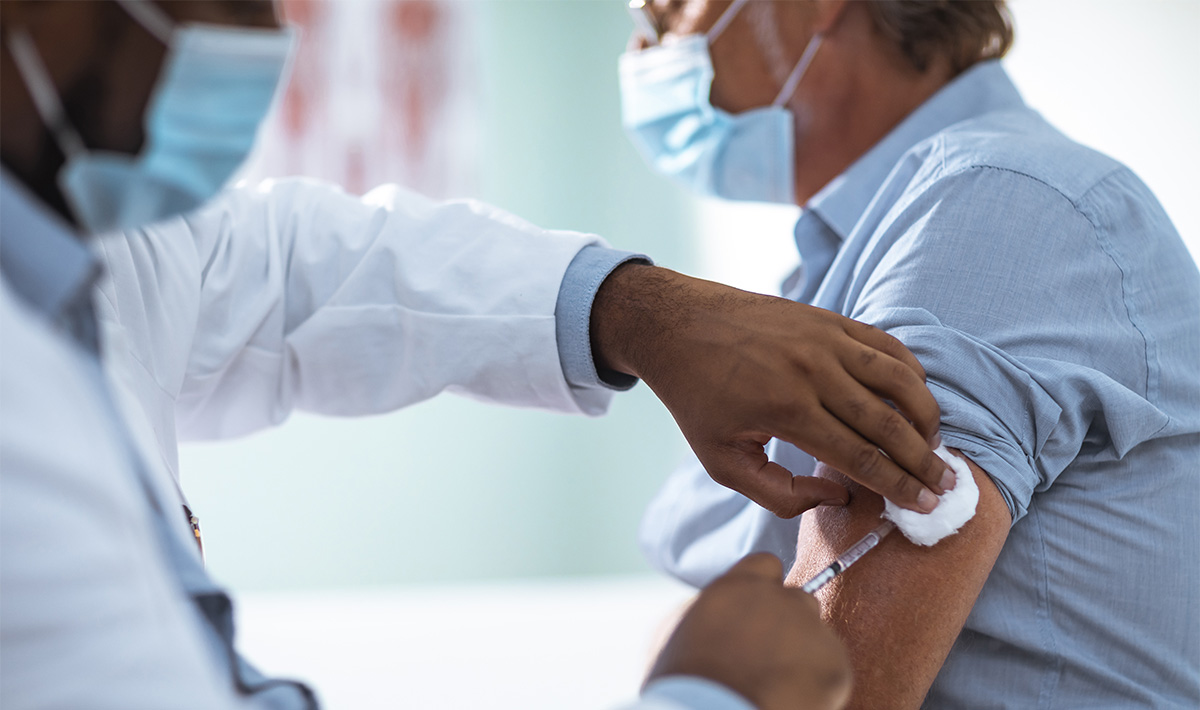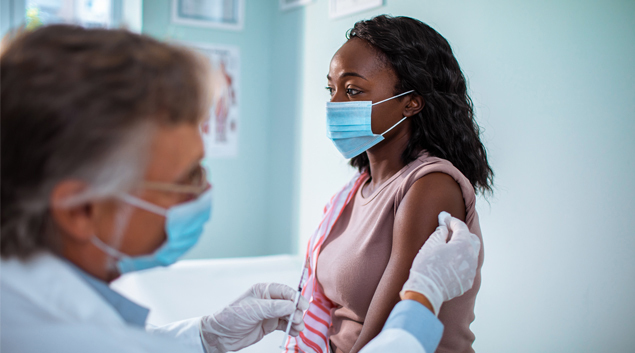
The Meals and Drug Administration’s advisory committee yesterday permitted the Moderna COVID-19 vaccine for emergency use authorization.
The vaccine now awaits approval by the FDA.
Yesterday, FDA Commissioner Dr. Stephen Hahn stated the FDA would quickly work towards finalization and issuance of an emergency use authorization. The company has additionally notified the U.S. Facilities for Illness Management and Prevention and Operation Warp Pace to allow them to execute their plans for well timed vaccine distribution, Hahn stated.
HIMSS20 Digital
Study on-demand, earn credit score, discover merchandise and options. Get Began >>
Shut to 6 million doses of the Moderna vaccine are anticipated to be distributed quickly, with 20 million going out by the tip of December, based on Operation Warp Pace officers.
Moderna is the second vaccine to obtain FDA advisory committee approval. The Pfizer vaccine, which the FDA permitted final week, is being given to frontline staff and is now, with the assistance of pharmacies, going out to employees and residents of long-term care services. A full-scale rollout to long-term care services is anticipated on Monday.
Not like the Pfizer vaccine, the Moderna vaccine doesn’t have to be stored at minus 70 levels Celsius and could be saved at common freezer temperatures.
Twitter: @SusanJMorse
E mail the author: susan.morse@himssmedia.com