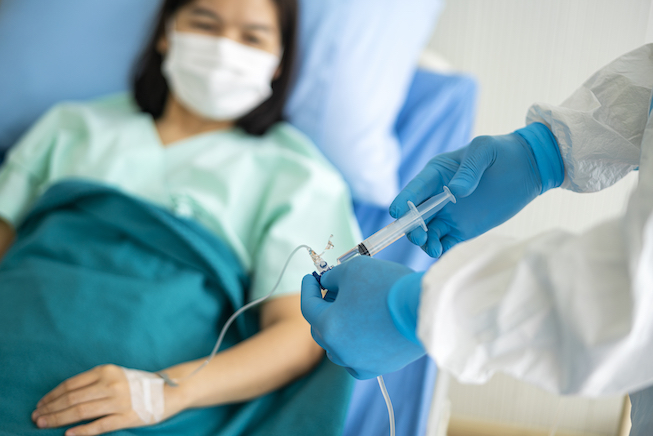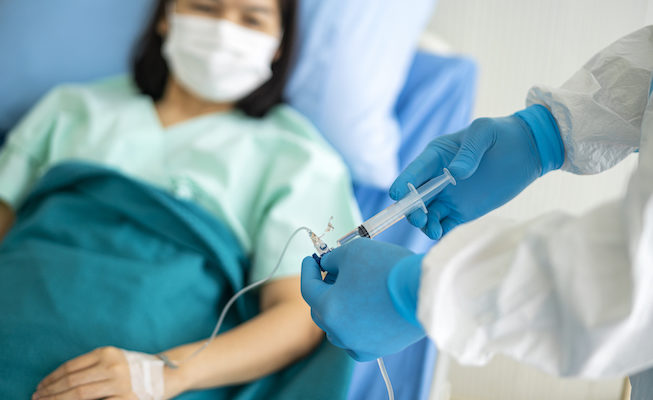
The U.S. Division of Well being and Human Companies has introduced plans to distribute Regeneron’s monoclonal antibody therapeutics, casirivimab and imdevimab, starting Tuesday.
Roughly 30,000 doses are able to be distributed with extra to be prepared within the coming weeks, in keeping with HHS Secretary Alex Azar.
HHS will allocate these doses to state and territorial well being departments which is able to then decide the healthcare amenities that can obtain the therapeutic. Weekly allocations shall be primarily based proportionately on confirmed COVID-19 circumstances and hospitalizations in every state and territory over the earlier seven days.
HIMSS20 Digital
Be taught on-demand, earn credit score, discover merchandise and options. Get Began >>
Attainable administration areas embody hospital outpatient amenities, hospital emergency departments, and alternate care websites arrange by hospitals and well being departments beneath the ‘hospital with out partitions,’ flexibility supplied by the Facilities for Medicare and Medicaid Companies to assist a surge of hospitalized sufferers.
The therapy shall be accessible for free of charge to sufferers, though healthcare amenities might cost for the administration.
WHAT’S THE IMPACT?
The Regeneron therapeutics acquired emergency use authorization from the U.S. Meals and Drug Administration on November 21, to be administered collectively for the therapy of gentle to average COVID-19 non-hospitalized adults and pediatric sufferers 12 years of age or older weighing at the very least 88 kilos who’re at excessive threat for extreme development.
A very powerful signal of efficacy of this therapy in medical trials was its means to decrease hospitalizations and emergency room visits amongst sufferers handled with it, in keeping with Azar.
Underneath the EUA, casirivimab and imdevimab have to be administered collectively – and in settings the place healthcare suppliers have speedy entry to drugs to deal with a extreme infusion response, comparable to anaphylaxis, and the power to activate the emergency medical system.
Amenities will need to have acceptable healthcare staffing, coaching, tools and area accessible to manage the remedy in a way that minimizes an infection transmission, HHS mentioned. Subsequently, it says that extra preparation time could also be required for some therapy areas earlier than they’ll administer the drug to sufferers.
THE LARGER TREND
That is now the third drug to be allowed to deal with COVID-19 by the FDA.
The primary was the antiviral drug Veklury (remdesivir) to be used in grownup and pediatric sufferers 12 years outdated and older and weighing at the very least 88 kilos for COVID-19 remedies requiring hospitalization. This drug ought to solely be administered in a hospital or in a healthcare setting able to offering acute care corresponding to inpatient hospital care, in keeping with the FDA.
The second was Eli Lilly and Firm’s investigational neutralizing antibody bamlanivimab, which is permitted for the therapy of gentle to average COVID-19 in adults and pediatric sufferers 12 years and older with a optimistic COVID-19 check who’re at excessive threat for progressing to extreme COVID-19 and/or hospitalization.
When President Trump was hospitalized with COVID-19, he was handled with remdesivir, the Regeneron therapeutics and several other different medication, together with zinc, vitamin D and the generic model of the heartburn therapy Pepcid.
The FDA has nonetheless but to approve a COVID-19 vaccine. Nevertheless, over the previous a number of weeks Pfizer and Moderna have launched efficacy outcomes from their respective trials displaying that every candidate is greater than 90% efficient. Final Friday, Pfizer submitted a request for EUA to the FDA for its candidate.
On Monday, AstraZeneca launched knowledge on its vaccine candidate that it has a median efficacy of 70%.
As soon as a vaccine is permitted by the FDA, officers from Operation Warp Velocity say distribution will start inside 24 hours.
ON THE RECORD
“Authorization and distribution of this new Regeneron antibody therapy is one other important step ahead in treating sufferers and bridging us to the rollout of secure and efficient vaccines, with all of those efforts made attainable by Operation Warp Velocity,” mentioned HHS Secretary Alex Azar. “Federal allocation of therapeutics like Regeneron’s, in cooperation with our state and native authorities companions, will assist be sure that they go to the sufferers who want them probably the most, simply days after the product is permitted.”