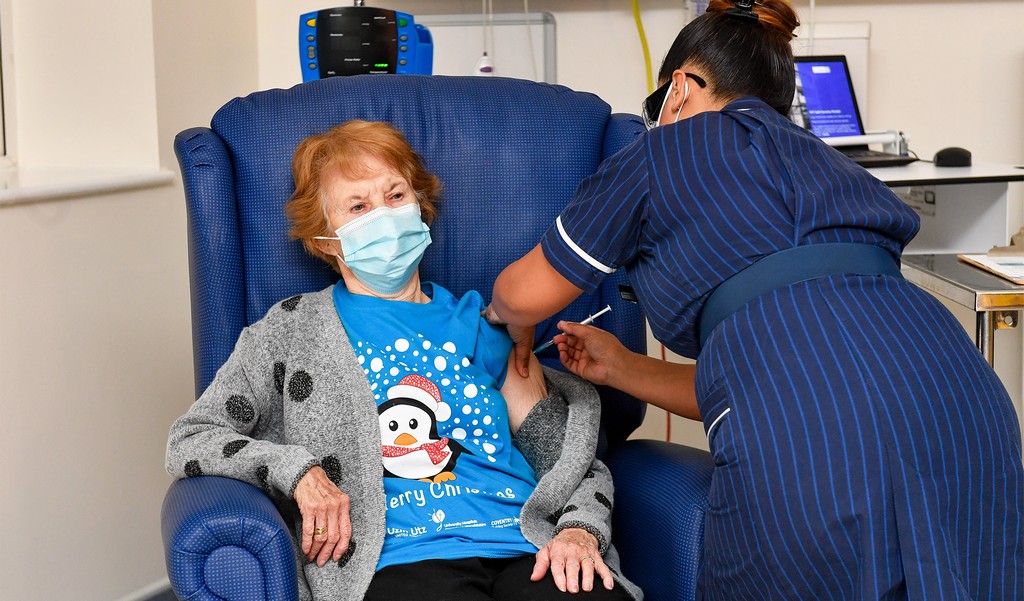
The Pfizer/BioNTech vaccine rollout continues in the UK after healthcare employees and aged people turned the primary on this planet to obtain the shot.
However after two healthcare employees suffered hostile reactions to the vaccine, Nationwide Well being Service England warned that folks with a “vital historical past of allergic reactions” shouldn’t be given the Pfizer/BioNTech coronavirus vaccine, in line with CNN. Each workers members reportedly had a major historical past of allergic reactions and carried adrenaline autoinjectors.
Most reactions have been delicate and have been in contrast to what’s skilled after getting a flu shot, comparable to a sore arm on the injection web site. Hundreds within the UK have been vaccinated on Tuesday.
HIMSS20 Digital
Study on-demand, earn credit score, discover merchandise and options. Get Began >>
The vaccine presents some safety after the primary dose, however two doses are required for full safety, in line with paperwork launched Tuesday by the Meals and Drug Administration, forward of Pfizer’s assembly with the FDA tomorrow. The Pfizer vaccine is reportedly 95% efficient in defending in opposition to the coronavirus.
In the US, Pfizer and Moderna are able to go along with their COVID-19 vaccines, in line with Operation Warp Velocity, with distribution to front-line employees and sufferers in long-term care services to be the primary on this nation to start out receiving the vaccines this month.
Each drug corporations have filed for emergency use authorization with the Meals and Drug Administration.
The FDA’s vaccine advisory committee will evaluate emergency use authorization for the Pfizer vaccine on Thursday, December 10, and the Moderna vaccine on Thursday, December 17.
See our persevering with vaccine protection right here:
Eli Lilly and UnitedHealth Group accomplice on COVID-19 antibody therapy for high-risk people
Operation Warp Velocity is trying to begin distributing COVID-19 vaccine by mid-month
Moderna seeks regulatory authorization for its COVID-19 vaccine
HHS will start allocating Regeneron’s COVID-19 therapeutic this week
HHS companions with chain and impartial pharmacies to extend entry to future COVID-19 vaccines
COVID-19 vaccine distribution will start inside 24 hours of an emergency use authorization
Moderna vaccine candidate reveals 94.5% efficacy in opposition to COVID-19
Pfizer touts new COVID-19 vaccine, calling it ‘90% efficient’
Medicare beneficiaries can get monoclonal antibody COVID-19 remedies for gratis
FDA grants emergency use authorization for Eli Lilly antibody therapy for COVID-19