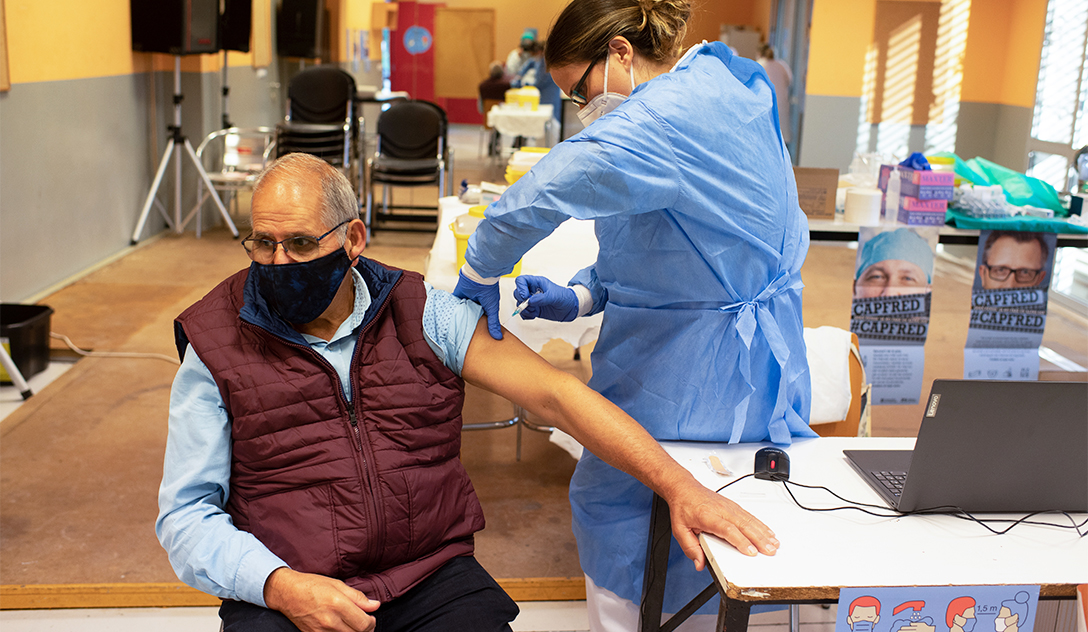
The advantages of the Pfizer-BioNTech COVID-19 vaccine outweigh its dangers in people 16 years and older, the Meals and Drug Administration’s Vaccines and Associated Organic Merchandise Advisory Committee voted on Thursday.
WHY THIS MATTERS
The vote, which was 17 in favor and 4 in opposition to, with one abstention, paves the way in which for the FDA to authorize the vaccine for emergency use, and, if accredited, to begin distribution by mid-month.
Dr. William C. Gruber, senior vice chairman, Vaccine Medical Analysis and Growth for Pfizer, stated the outcomes of the scientific trials had been encouraging. There was no proof of enhanced illness, he stated. Efficacy was excessive throughout racial teams.
HIMSS20 Digital
Be taught on-demand, earn credit score, discover merchandise and options. Get Began >>
Ache on the vaccine website was largely delicate and reasonable, with similar to reactions to different vaccines, he stated.
CONCERNS
The committee met for a full 8 hours, with their choice seen as a foregone conclusion, based mostly on an FDA briefing doc launched days earlier than the assembly that confirmed the vaccine was protected and 95% efficient.
Nevertheless, committee members expressed considerations in regards to the Pfizer trial.
An unidentified variety of contributors developed Bell’s Palsy, a situation that causes non permanent weak spot or paralysis within the muscle tissue of the face. Committee member Dr. Susan Wollersheim, medical officer, Division of Vaccines and Associated Merchandise for the FDA, stated there have been such small numbers of contributors who developed Bell’s Palsy that it might be troublesome to narrate the situation to the vaccine at the moment.
One other concern was whether or not 16- and 17-year-olds ought to have the ability to get vaccinated once they weren’t a part of the trial.
The incidents of extreme COVID-19 at this age will not be excessive, stated member Dr. Ofer Levy, a employees doctor who’s a part of the Precision Vaccines Program at Boston Youngsters’s Hospital and a professor at Harvard Medical College. However when you take away the 16- and 17-year-olds, you lose the trouble to “climb down in age and conquer the pandemic,” he stated.
Others stated the information on this age group was too skinny, since the scientific trial didn’t embrace youngsters. The U.S. examine was made up of 19,000 adults, ages 18 to 55 and 65 to 85, who had been evenly break up between getting a placebo and the vaccine.
The contributors had been swabbed to find out they weren’t contaminated prior to every dose. The Pfizer vaccine requires two doses.
Different committee members questioned the allergic response by two healthcare employees who got the vaccine in England and the size of time of its effectiveness.
Evan Fein, a Part I trial participant, stated he believed he acquired the true vaccine and never a placebo, as a result of he had fever and chills. He was referred to as repeatedly by medical doctors to see if he was all proper, he stated. As to the query of what occurs in the long run, Fein stated that after 5 months he had no long-term results.
RECOMMENDATIONS
Some committee members really useful that Pfizer preserve the trial going, even because the vaccine is being distributed, and to make it possible for contributors who obtained the placebo don’t get neglected of being given an precise vaccine.
Sidney Wolfe of residents.org requested whether or not the placebo topics needs to be advised of their standing. If not, they could depart the trial to get vaccinated, he stated.
All trial information must be made public, committee members stated.
THE LARGER TREND
The advisory committee is scheduled to undergo an identical overview of the Moderna vaccine for EUA authorization on Thursday, December 17.
As a vaccine turns into obtainable, greater than a 3rd of adults say they’re unwilling to take it, in accordance with the Worker Profit Analysis Institute – a personal, nonpartisan, nonprofit analysis group – and unbiased analysis agency Greenwald Analysis, which launched their annual Shopper Engagement in Well being Care Survey.
The CEHCS discovered that solely 55% of the grownup inhabitants was prepared to obtain a COVID-19 vaccine, and 24% stated they might not get the vaccine. 9 p.c stated that it relies upon, and 12% had been uncertain.